PEDIATRICS
Optimize study design for pediatric patients.
Improve study outcomes for pediatric therapies by addressing the needs of children and their families –
from formulations to biospecimen requirements.
Improve study outcomes for pediatric therapies by addressing the needs of children and their families –
from formulations to biospecimen requirements.




Pediatric expertise is essential to developing and commercializing pediatric therapies, from the earliest planning stages through launch and real-world assessment. We provide a range of scientific, therapeutic, regulatory, and operational expertise to ensure study success.
These resources extend beyond IQVIA to encompass a global network of top performing sites, ensuring there are sites around the world with the proven ability to conduct every pediatric trial.
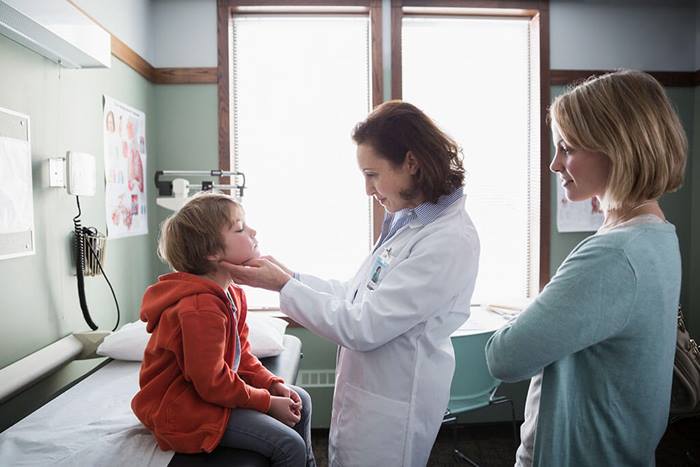
Successful pediatric trial designs are attentive to the needs of infant, child, or adolescent patients. And decision making about study participation typically involves multiple family members.
IQVIA considers the differences within pediatric sub-populations and takes a sensitive approach to these patients and their caregivers.


The RACE for Children Act will have significant impact on cancer drug development. Among all ongoing cancer trials, more than 70% involve RACE-defined molecular target drugs, but only 6.9% appear to include pediatric-age participants, according to a new analysis conducted by the IQVIA Institute.
Build on our experience of more than 245 rare disease studies in 96 countries to fulfill your promise of hope to millions around the world.
Identify the best performing sites for your trial in less time with new machine learning and predictive modeling methods.
Bring trials directly to patients to improve access and engagement, increase quality and shorten timelines.